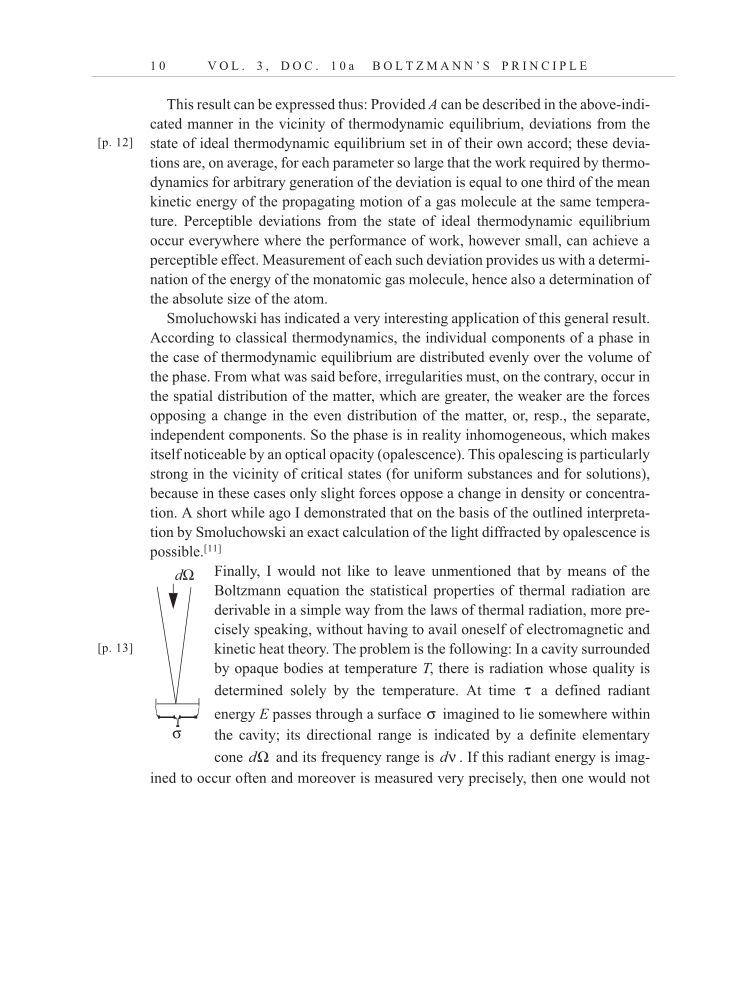
1 0 V O L . 3 , D O C . 1 0 a B O L T Z M A N N ’ S P R I N C I P L E This result can be expressed thus: Provided A can be described in the above-indi- cated manner in the vicinity of thermodynamic equilibrium, deviations from the state of ideal thermodynamic equilibrium set in of their own accord these devia- tions are, on average, for each parameter so large that the work required by thermo- dynamics for arbitrary generation of the deviation is equal to one third of the mean kinetic energy of the propagating motion of a gas molecule at the same tempera- ture. Perceptible deviations from the state of ideal thermodynamic equilibrium occur everywhere where the performance of work, however small, can achieve a perceptible effect. Measurement of each such deviation provides us with a determi- nation of the energy of the monatomic gas molecule, hence also a determination of the absolute size of the atom. Smoluchowski has indicated a very interesting application of this general result. According to classical thermodynamics, the individual components of a phase in the case of thermodynamic equilibrium are distributed evenly over the volume of the phase. From what was said before, irregularities must, on the contrary, occur in the spatial distribution of the matter, which are greater, the weaker are the forces opposing a change in the even distribution of the matter, or, resp., the separate, independent components. So the phase is in reality inhomogeneous, which makes itself noticeable by an optical opacity (opalescence). This opalescing is particularly strong in the vicinity of critical states (for uniform substances and for solutions), because in these cases only slight forces oppose a change in density or concentra- tion. A short while ago I demonstrated that on the basis of the outlined interpreta- tion by Smoluchowski an exact calculation of the light diffracted by opalescence is possible.[11] Finally, I would not like to leave unmentioned that by means of the Boltzmann equation the statistical properties of thermal radiation are derivable in a simple way from the laws of thermal radiation, more pre- cisely speaking, without having to avail oneself of electromagnetic and kinetic heat theory. The problem is the following: In a cavity surrounded by opaque bodies at temperature T, there is radiation whose quality is determined solely by the temperature. At time a defined radiant energy E passes through a surface imagined to lie somewhere within the cavity its directional range is indicated by a definite elementary cone and its frequency range is . If this radiant energy is imag- ined to occur often and moreover is measured very precisely, then one would not [p. 12] dΩ σ [p. 13] τ σ dΩ dν