DOC.
13
ELASTIC BEHAVIOR AND SPECIFIC HEAT
335
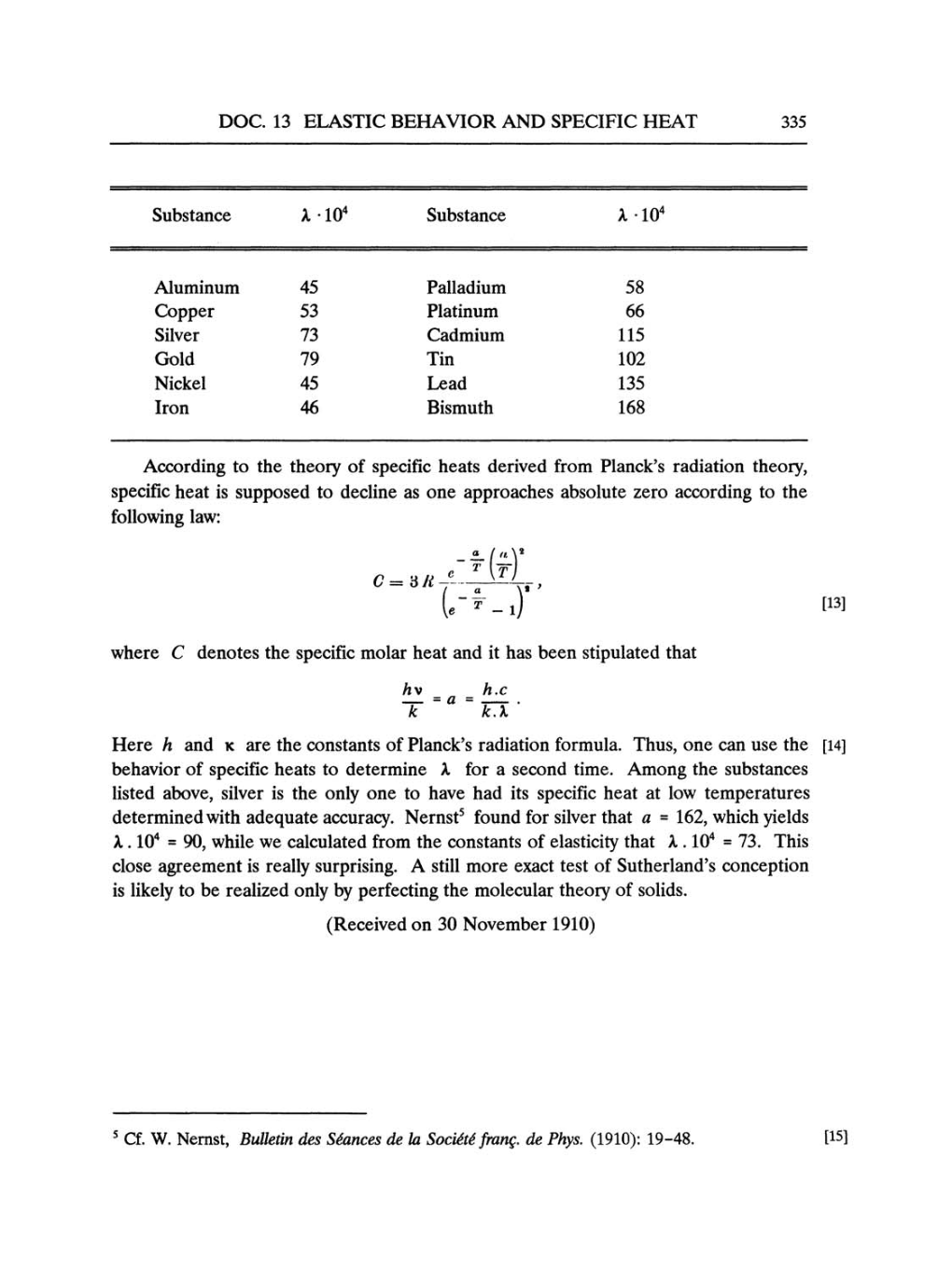
Substance A.104
Substance
X
.
104
Aluminum
45
Palladium
58
Copper
53
Platinum
66
Silver
73
Cadmium
115
Gold
79
Tin
102
Nickel 45
Lead
135
Iron
46
Bismuth
168
According
to
the
theory
of
specific
heats derived from Planck's radiation
theory,
specific
heat
is
supposed
to decline
as one approaches
absolute
zero according
to
the
following
law:
(
a
\
2
T
C
=
'6
H
[13]
where
C
denotes the
specific
molar heat and
it has
been
stipulated
that
hV
_
h.c
T
=a
=
EX
Here h and
k
are
the
constants
of
Planck's
radiation
formula.
Thus, one
can
use
the
[14]
behavior of
specific
heats
to
determine
X
for
a
second
time.
Among
the
substances
listed
above,
silver
is
the
only one
to have
had
its
specific
heat
at low
temperatures
determined
with
adequate
accuracy.
Nernst5
found for
silver
that
a
=
162,
which
yields
X.
104
=
90,
while
we
calculated
from
the
constants
of
elasticity
that
X
.
104
=
73.
This
close
agreement
is
really surprising.
A
still
more
exact test
of Sutherland's
conception
is
likely
to
be realized
only by
perfecting
the molecular
theory
of
solids.
(Received
on
30
November
1910)
5
Cf. W. Nernst, Bulletin des Seances de la Societe
franc.
de
Phys.
(1910):
19-48.
[15]