92
DOC.
127
NOVEMBER
1908
Number of molecules N
=
n
=
number of
free,
negative
electrons
(with oo
small
radius)
that
are
also
present.
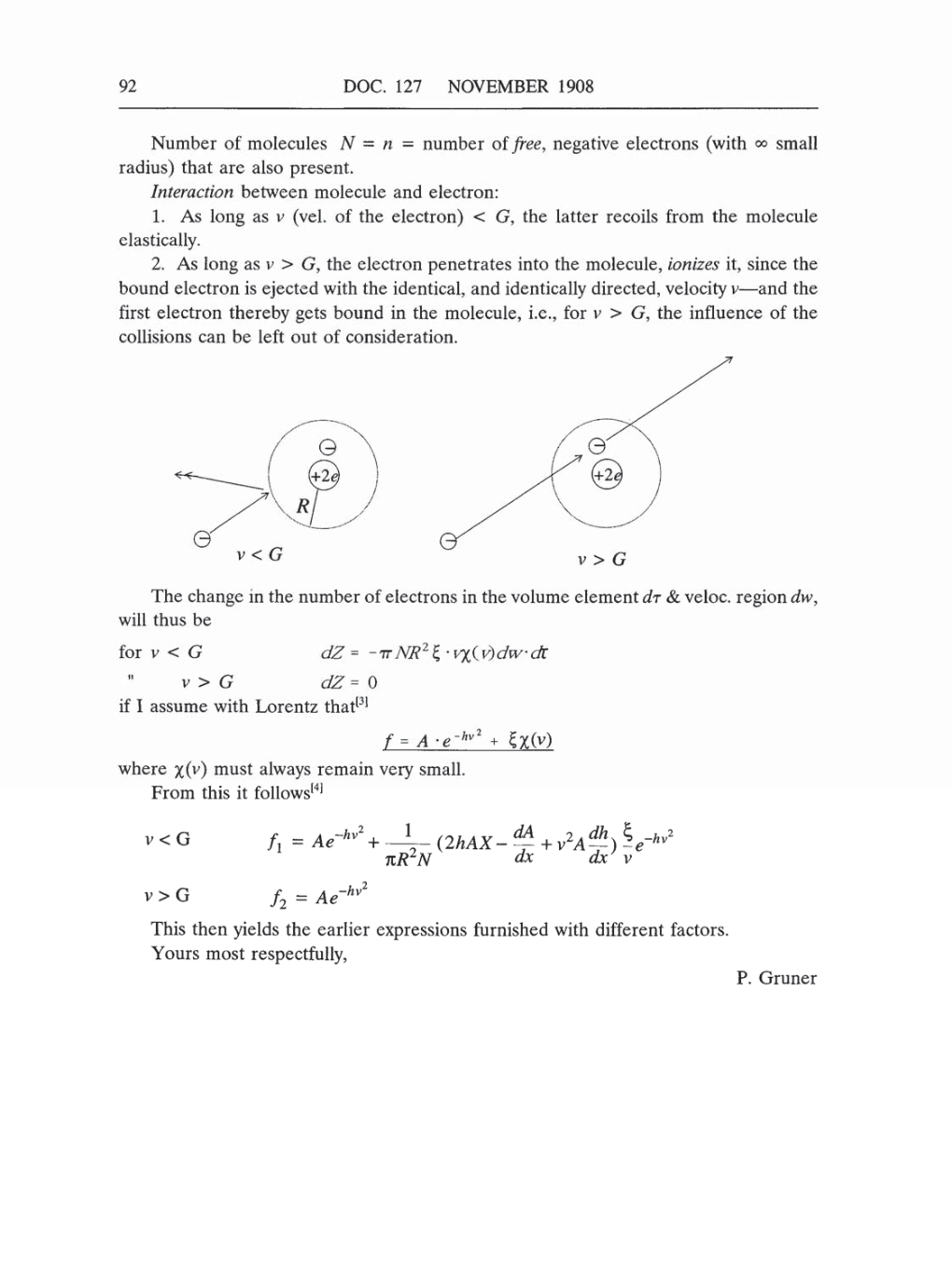
Interaction
between
molecule and
electron:
1.
As
long
as v
(vel.
of the
electron) G,
the
latter
recoils from
the
molecule
elastically.
2.
As
long as v
G,
the electron
penetrates
into the
molecule,
ionizes
it,
since
the
bound electron
is
ejected
with
the
identical,
and
identically
directed,
velocity
v-and the
first
electron
thereby
gets
bound
in the
molecule, i.e.,
for
v
G,
the influence of the
collisions
can
be left
out
of consideration.
The
change
in
the number of electrons
in the volume
element dr
&
veloc.
region
dw,
will thus be
v
G
for
v
G
v
G
dZdZ
=
0
if I
assume
with Lorentz
that[3]
dZ
=
-irNR2^
-vx(v)dw.dc
=
0
f
=
A.e-hv2
+
gX(v)
where
X(v)
must
always
remain
very
small.
From
this it
follows[4]
v
G
f1
=
Ae-hv2 +
1-L-
(2hAX-dA+V2Adh-dx)e-hv2dx
nR
N
v
G
f2
=
Ae~hv2
This
then
yields
the earlier
expressions
furnished
with
different
factors.
Yours
most
respectfully,
P.
Gruner