10
DOC.
6
FEBRUARY
1903
That the
solubility-to
some
extent-of
almost all solid
substances
in all
liquids
indicates
that
individual
molecules
possess high
velocities
even
in solid substances. This
corresponds
to
the
phenomena
in "solid
solutions."[17]
One
might try
to
apply
the
correspondingly
modified Maxwell
velocity
distribution
law to
quantitative
investigations.
That, in
any
case, gravitation
does
not
seem
to
suffice
as
attractive
force[18]-since
it
seems,
at
least
at
first
sight,
that the
liquids
with
greatest
specific
weight
would
then
have to
display
the
greatest
dissolving
and dissociative
ability.
But
changes
of volume
should
not be
ignored,
because
they
are
essential
for the
"potential gradient"; i.e.,
if
solution
or
dissociation
are accompanied
by a
reduction of the total
volume,
then
gravitation
performs
work
by
this
very
act,
and
vice
versa.
To
sum up:
I
consider the
formulation
of
the
condition
for
dissociation
given on
p.
1,
and
the
corresponding
condition
for
solution, to
be
new.
They
render the "ion
hydrates"
probable (even
necessary)
and
the
disagreement
between Ostwald's dilution
law and
experience harmless.[19]
Finally,
the "ion
hydrate theory"
allows
us
to
retain Sutherland's
hypothesis
for
the
time being-only
as a
tool,
of
course,
because,
in
general,
solution
phenomena,
that
is
to
say,
in
my
opinion,
chemical forces
play
a
part
in
semipermeable
membranes-and
to
draw
inferences
with
regard to
incompletely
semipermeable
membranes.
Finis,
and
my
best
regards!
Michele
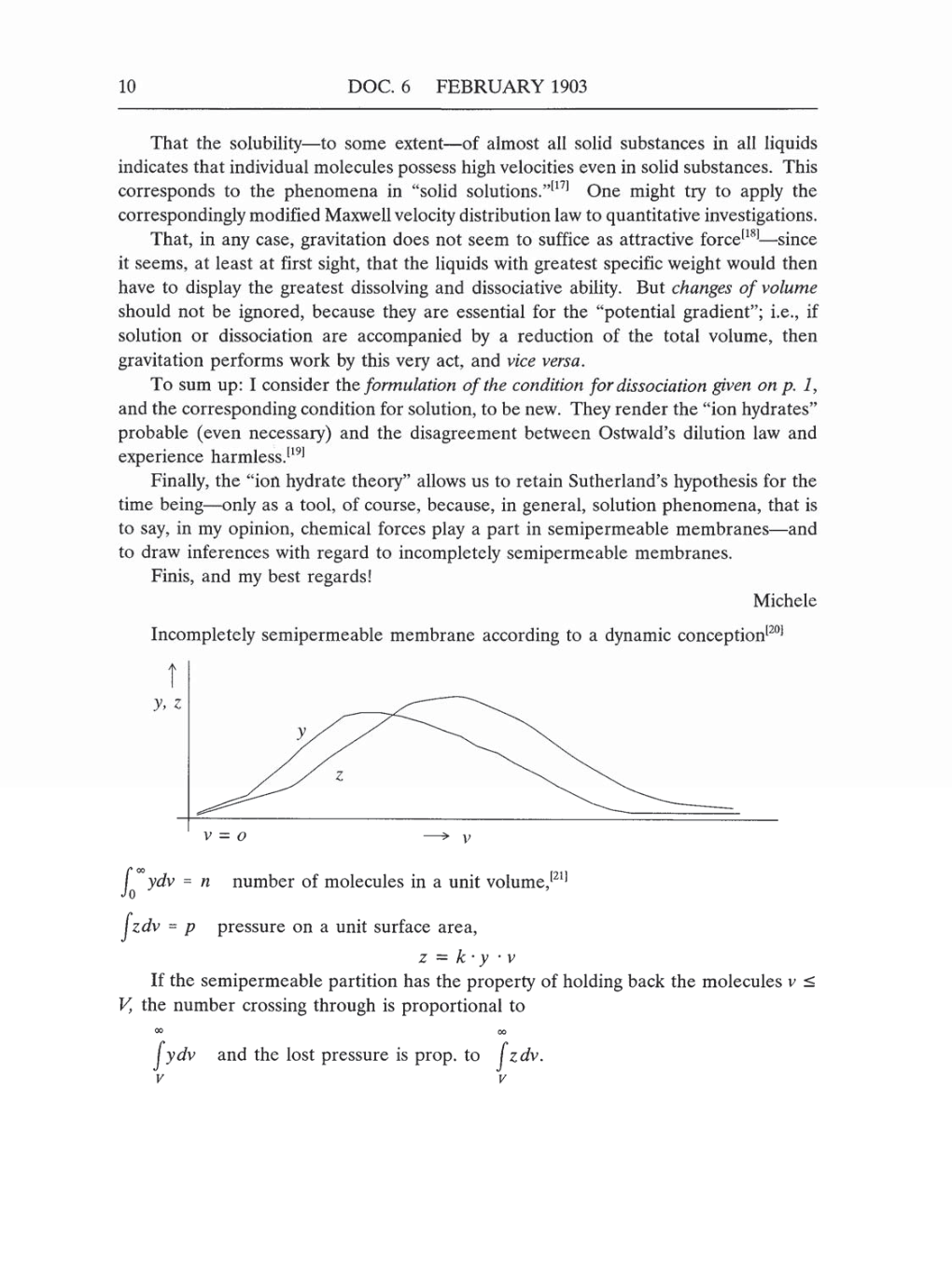
Incompletely semipermeable
membrane
according
to
a
dynamic
conception[20]
0ydv =
n
number of molecules
in
a
unit
volume,[21]
Jzdv
=
p
pressure
on a
unit
surface
area,
z
=
k
·
y
·
v
If the
semipermeable partition
has the
property
of
holding
back
the molecules
v
V,
the
number
crossing
through is proportional
to
J
ydv
and the
lost
pressure is prop.
to
J
zdv.
v
v