4.
Lecture Notes for Course
on
the
Kinetic
Theory
of Heat
at
the
University
of
Zurich,
Summer
Semester
1910
[19
April-5
August
1910][1]
u
i
u2 u3
-
u1
u2 u3
-
=
k
K
=
V
-
k
=
p
dt ^
[2]
[fly-leaf]
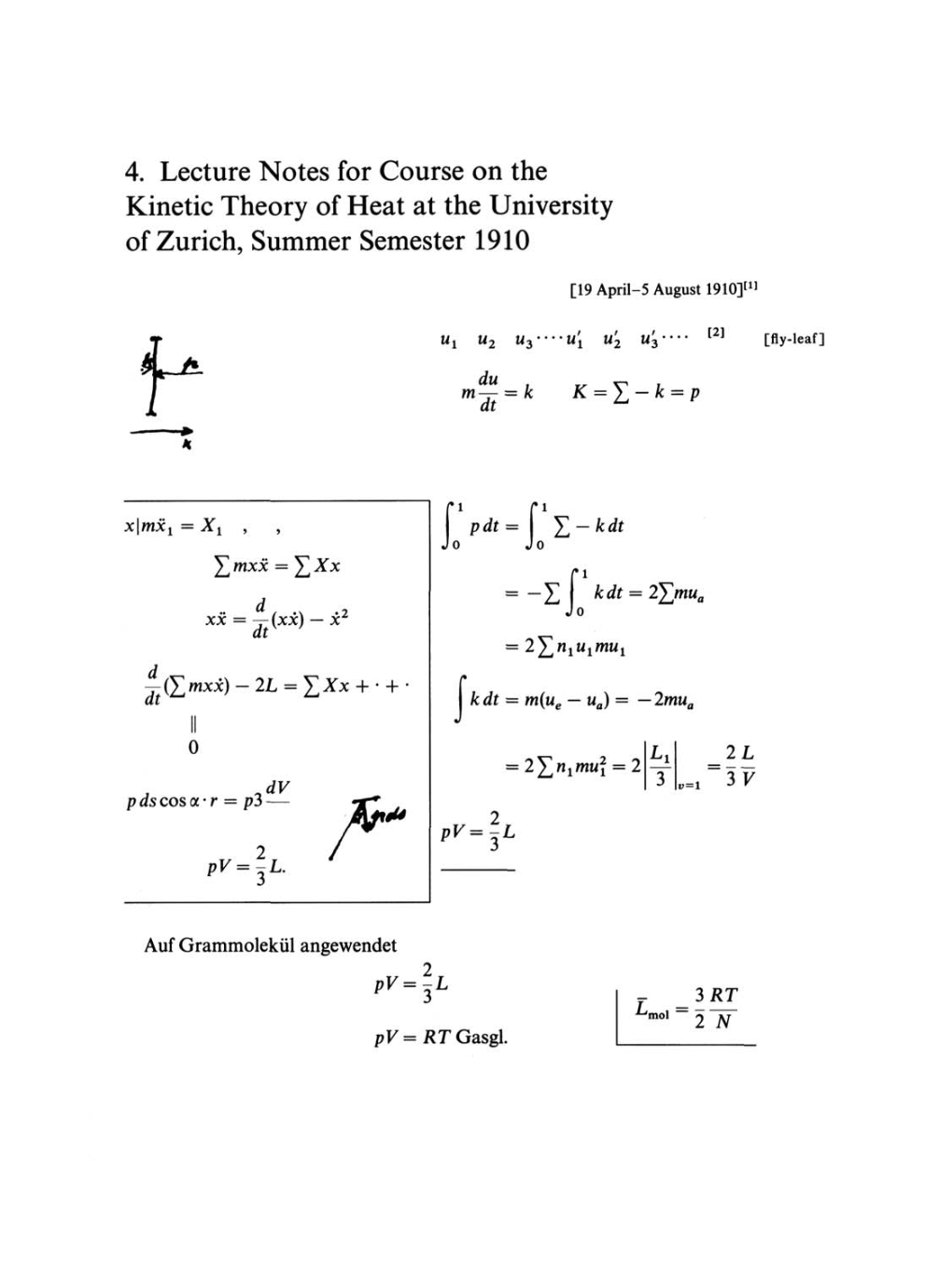
xlmx!
=
X1
, ,
£mxx
=
£^x
xx
=
-y-
(xx)
-
x2
dt
d
dt
(£mxx)
-
2
L
=
YjXx
+
'
+
0
p
ds
cos
a
•
r
=
p3
dV
?V=-,L.
$U
pdt
=
o
r i
o
X
-
kdt
=
~X
I
kdt
=
2Y,mu,
0
=
2^M1M1wm1
kdt
=
m(ue
-
ua)
=
-2mu,
=
2Yjn1mu\
=
2
Li
3
U
= 1
2
L
3
K
pV
=
2/3L
Auf Grammolekül
angewendet
2
pK-jL
pV
=
RT
Gasgl.
^mol
3
.RT
2
JV